MSL Blog post
A composite endpoint in a clinical trial

A composite endpoint is an endpoint that is a combination of multiple clinical endpoints for example: death, myocardial infarction or death in cardiovascular clinical trials. Composite endpoints can be primary or secondary.
The benefits of composite endpoints are:
- Increased statistical efficiency and precision.
- Sm...
What drives the cost of clinical trials
The total cost of a clinical trial depends on many variables, but the main cost factors are:
- the number of participants, and
- the complexity of the clinical trial protocol.
Firstly, the number of participants in a clinical trial is highly related with the study phase. For instance, phase 1 clinical trials —whic...
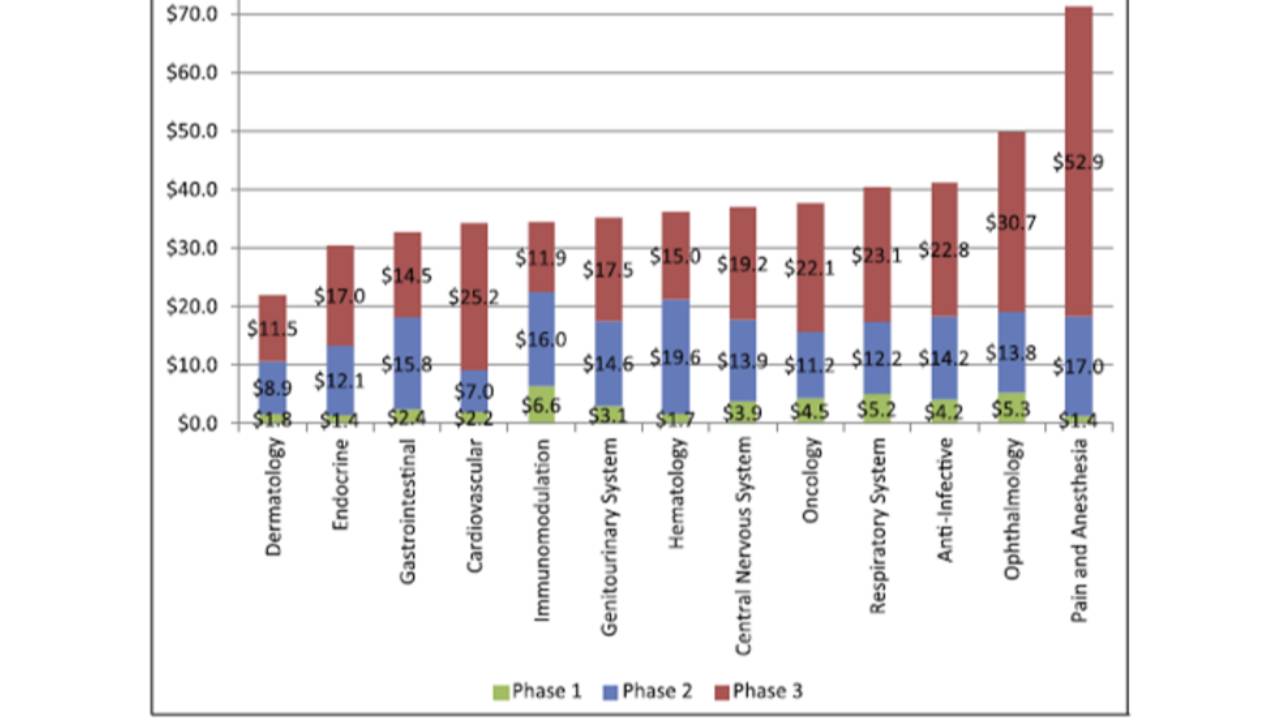
The costs of clinical trials
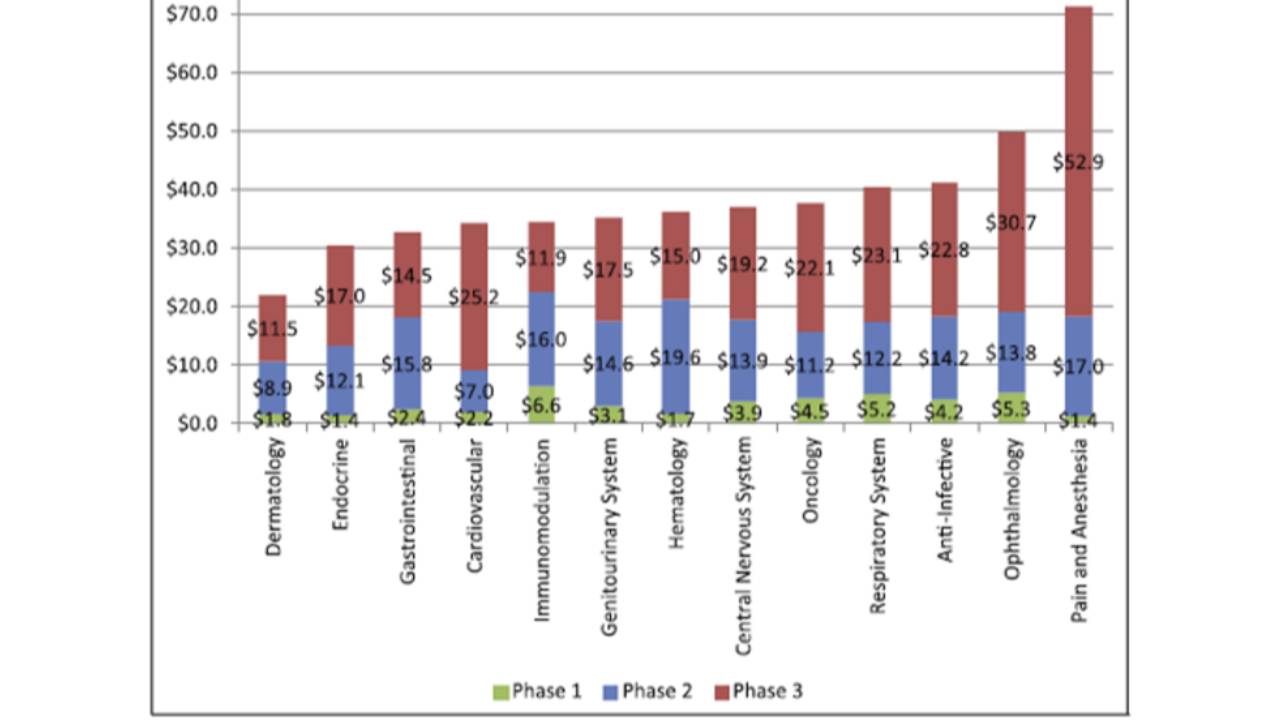
The average cost of a phase 1 study conducted at a United States clinical site ranges from US$1.4 million to US$6.6 million, including estimated site overhead and monitoring costs [1].
A phase 2 study in the U.S. costs from US$7.0 million (cardiovascular) to US$19.6 million (hematology) [1].
Phase 3 clinical trials...
Drug Development Takes Time
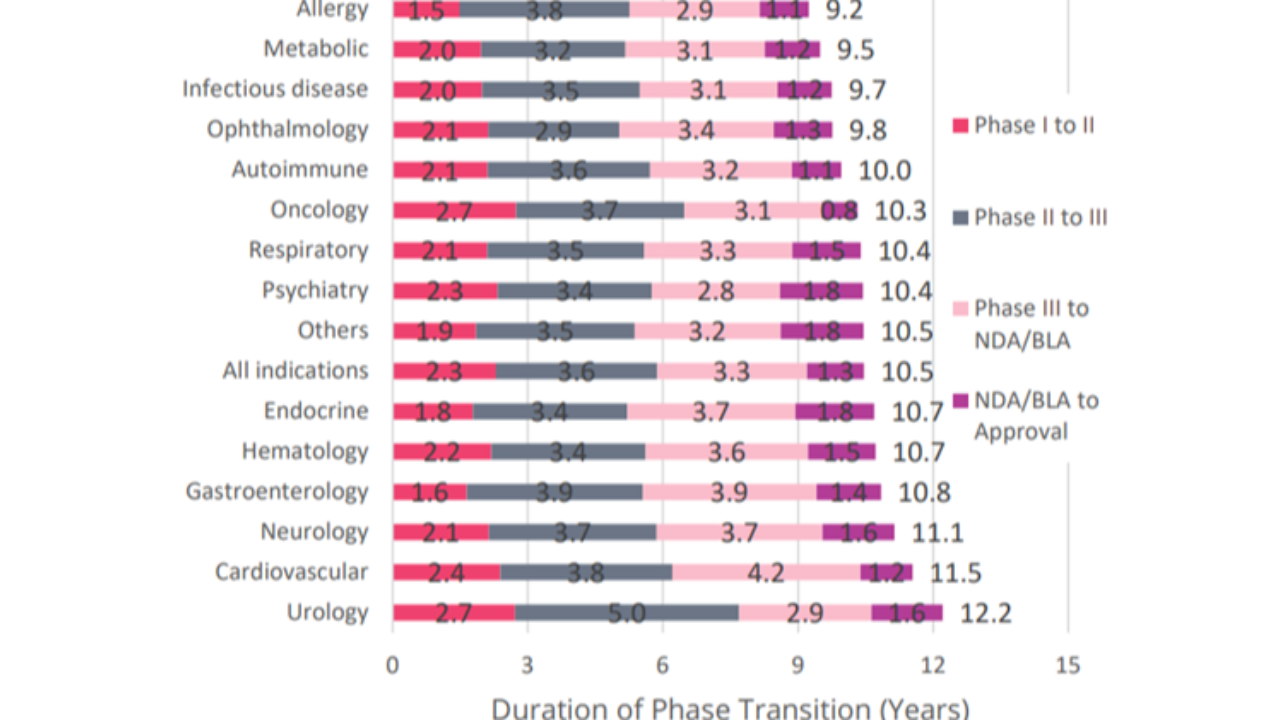
Based on 6,151 successful phase transitions over the 2011–2020 period, it took an average of 10.5 years for a drug to successfully progress from Phase I development to regulatory approval.
Key Takeaways.
These are valuable metrics as there are considerable opportunity costs associated with investing in an R&D proces...
Clinical trials audiobook for MSLs

Conducting clinical trials is essential in bringing a drug to the market. As an MSL you need to know everything about clinical trials, as trial read outs and trial data is what you will be discussing as an MSL with your KOLs.
FSTP has developed the first FREE audiobook on Clinical Trials together with Fast Facts/Karge...




