Drug Development Takes Time
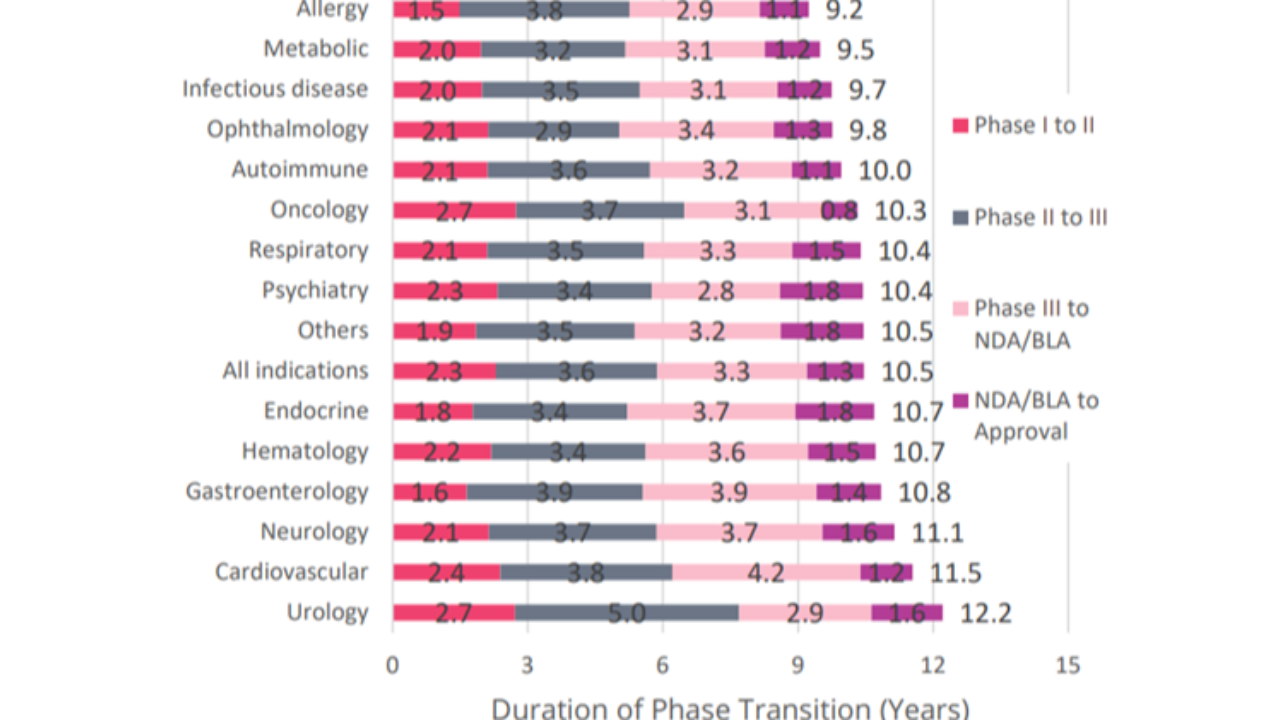
Based on 6,151 successful phase transitions over the 2011–2020 period, it took an average of 10.5 years for a drug to successfully progress from Phase I development to regulatory approval.
Key Takeaways.
These are valuable metrics as there are considerable opportunity costs associated with investing in an R&D process that may take up to a decade, and a simple analysis of clinical trial durations excludes the contribution of internal decision making and strategic execution from the overall timeline.
Phase duration can vary greatly according to numerous factors, such as disease area and indication, best practices of clinical trial design, and patient availability. This is the average duration across 14 common diseases:
- Average of 2.3 years at Phase I
- Average of 3.6 years at Phase II
- Average of 3.3 years at Phase III
- Average of 1.3 years at the regulatory stage.
- A total of 10.5-year average development duration!
Disease areas with above-average LOAs tend to be associated with shorter development timelines. Five of the seven best-performing disease areas by LOA fall beneath the 10.5-year average development duration, including all four groups with a duration of less than 10 years (#Allergy, #Metabolic, #Infectiousdiseases, and #Ophthalmology).
Conversely, the remaining disease areas, with below average LOAs, either have durations that lie very close to the 10.5-year average duration, or in the case of #Urology, #Cardiovascular and #Neurology, notably exceed the average.
Within each individual phase, there are some noteworthy performers.
#Oncology shares the longest Phase I transition at 2.7 years, it is also the only disease area to have an average regulatory review of less than 1 year; the 0.8-year duration is almost half as short as the cumulative total for all non-Oncology indications (1.4 years).
#Urology drug candidates experience the longest Phase II transition (5.0 years).
#Ophthalmology emerges as the fastest disease area for Phase II research (2.9 years).
When looking at Phase III timelines, Cardiovascular drug programs have the longest duration, extending to 4.2 years. The large cardiovascular patient population sizes in trials and the long-term evaluation of cardiovascular outcomes contribute to longer timelines than seen in other highly prevalent diseases, such as Psychiatry (2.8 years), which typically assesses short-term symptomatic improvement using rating-scale questionnaires.
Have you joined the MSL and medical affairs LinkedIn community yet? Over 10,000 med. affairs professionals across the globe sharing pharma news, MSL jobs, tips and actively networking.
This is the link: https://lnkd.in/gZ_mBprH
See you there,
Sue
Source: https://lnkd.in/gbdCQGkU
#MSL #MSLinterviewProcess #AspiringMSL #MSLTraining #BoardcertifiedMSL #BCMSLcert #BCMSL #MedicalAffairs #pharma #MD #PharmD #MSc #Pharmacist #Pharmacists #Postdoc #postdocs #mslsociety #pharmaceuticals @Kevin Mero




